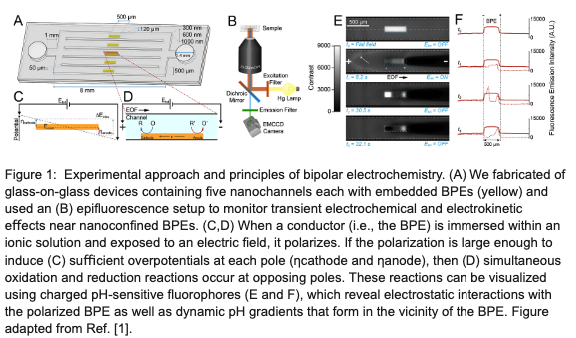
To probe the behavior of nanoconfined bipolar electrodes, we have performed preliminary experiments in which we monitored electrochemical dynamics in real-time using charged fluorophores. Through analysis of fluorescence enhancement and pH-sensitive quenching events, our results indirectly reveal the electrokinetic and electrochemical phenomena that occur near a nanochannel-confined BPE as it becomes charged, reaches a quasi-steady operating state, and then discharges upon the removal of an applied field. Specifically, we demonstrate that the removal of this field results in solution-phase charge imbalances within the EDL charge clouds surrounding the BPE poles. These imbalances induce intense and short-lived nonequilibrium electric fields that drive rapid and observable ion transport toward specific BPE locations as the bipolar EDLs dissipate and the system returns to equilibrium. By systematically varying the nature of the fluorophore, the concentration of the electrolyte, the strength of the applied field, and extent of oxide growth on the BPE surface, we are able to dissect the ion transport and heterogeneous charge transfer events that occur in the aftermath of field-induced polarization. The results contained in this work provide new insights into transient bipolar electrokinetics that improve our understanding of existing analytical platforms and can drive the development of novel micro- and nanoelectrochemical systems [1].
Reference:
[1] Scida, K., Eden, A., Arroyo-Currás, N., MacKenzie, S., Satik, Y., Meinhart, C.D., Eijkel, J.C.T., Pennathur, S. (2019). Fluorescence-Based Observation of Transient Electrochemical and Electrokinetic Effects at Nanoconfined Bipolar Electrodes. ACS Applied Materials and Interfaces, 11 (14), 13777-13786.