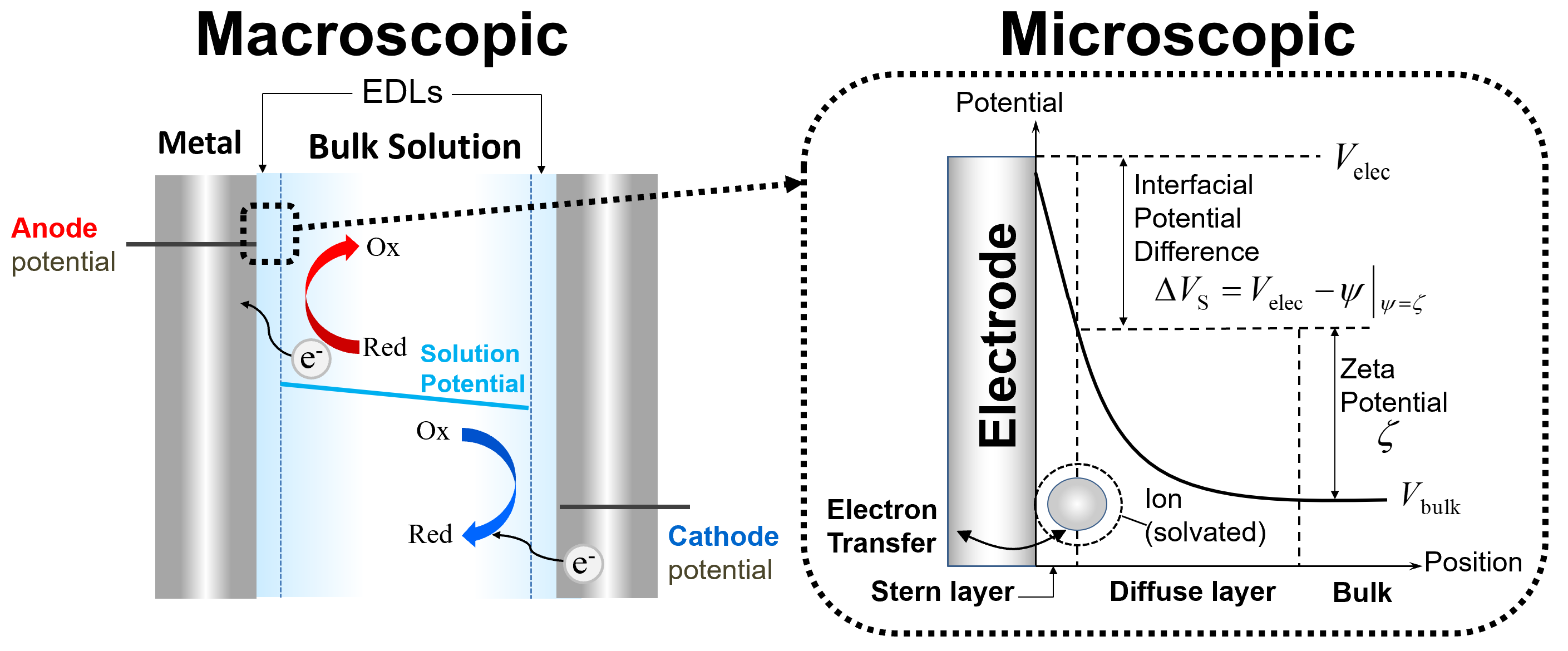
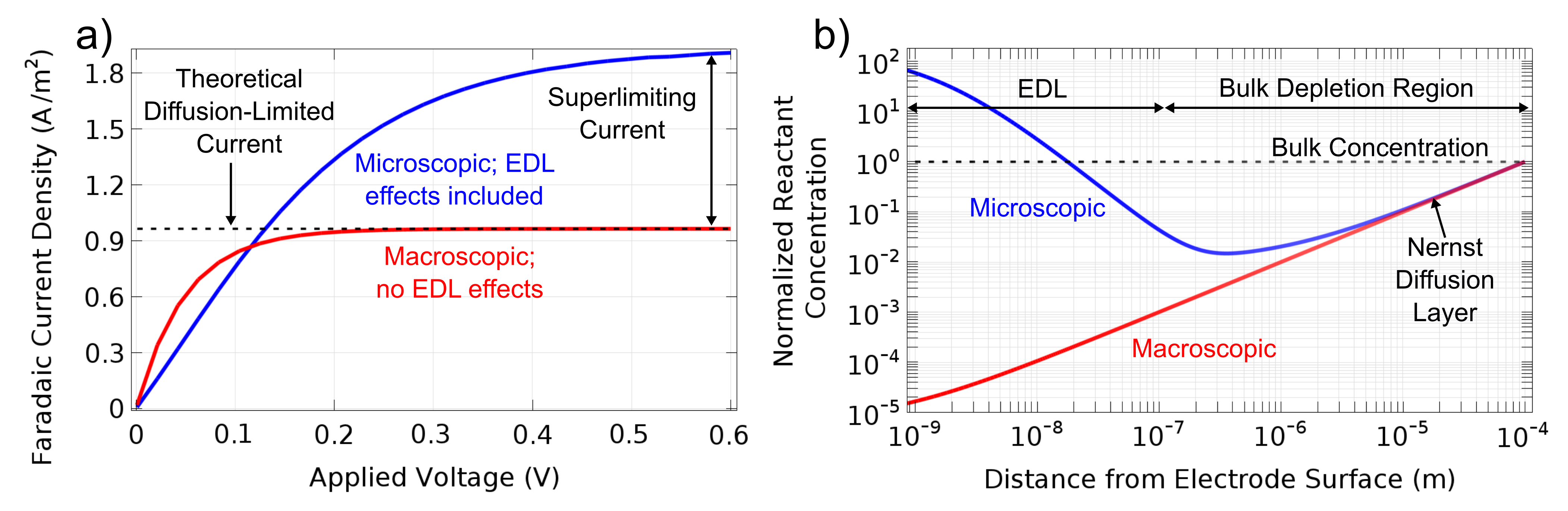
The structure of the EDL directly affects the kinetics of electrochemical reactions that occur when a polarized electrode is directly in contact with an ionic solution. Pioneering work by Alexander Frumkin [1] nearly a century ago revealed two major findings on this front: 1) electrostatic interactions between polarized electrodes and charged redox-active species alter reactant concentrations at the reaction plane, and 2) diffuse charge screening and capacitive EDL effects alter the effective driving potential of a redox reaction. Modifications such as the Frumkin correction have been introduced to account for EDL effects on heterogeneous charge transfer rates, but these approaches are limited to describing idealized EDLs with Boltzmann-distributed ions which remain in thermal equilibrium. In real electrochemical systems, however, EDLs can be perturbed from equilibrium if sufficient current is passed across the conductor/electrolyte interface [2]. Moreover, finite-sized EDLs in nanochannels and substantial reaction-induced changes in redox species concentrations at the electrode can lead to local deviations from theoretical “bulk” conditions of electroneutrality and uniform conductivity. In some cases, this results in the formation of nonequilibrium space charge regions which can extend considerable distances away from the electrode surface to generate ion concentration polarization zones. Hence, it is important that our nanoscale numerical models resolve diffuse charge screening effects and include the aforementioned findings of Frumkin in order to accurately analyze the intimately coupled electrokinetic and electrochemical dynamics within confined systems [3].
References:
[1] Frumkin, A. (1933). Hydrogen overpotential and the double layer structure. Zeitschrift für Physikalische Chemie, 164A (1), 121−133.
[2] Vetter, K.J. Electrochemical Kinetics: Theoretical and Experimental Aspects; Academic Press, Inc.: New York, 1967.
[3] Eden, A., Scida, K., Arroyo-Currás, N., Eijkel, J.C.T., Meinhart, C.D., Pennathur, S. (2019). Modeling Faradaic Reactions and Electrokinetic Phenomena at a Nanochannel-Confined Bipolar Electrode. Journal of Physical Chemistry C, 123 (9), 5353-5364.