Research
Experimental Electrokinetic Nanofluidics
The electric double layer (EDL) at the interface between electrolytes and charged surfaces significantly affects the performance of fuel cells, batteries, biologically-relevant ion-selective channels, supercapacitors, desalination, other electrokinetic, and electrochemical and bioanalytical processes. Comparing with the microscale, researchers are still studying fundamental details of the EDL in nanofluidic channels, especially in the case of multivalent and finite ion sizes. Knowing that the thickness of EDL depends on the concentration of the solution and the surface charge.
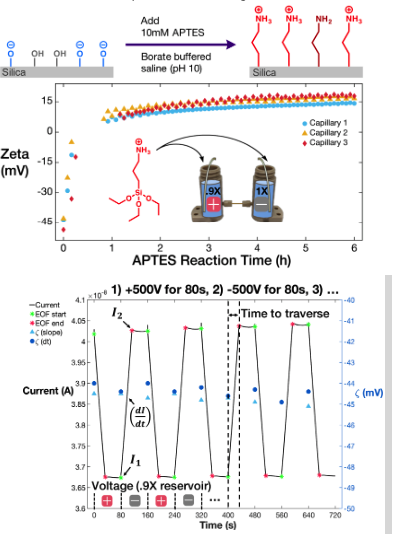
The ability to monitor chemical surface reactions precisely and in real-time can provide fundamental insights to help us create highly relevant and complex nanoscale technologies (e.g. cell surface engineering, surface-anchored biosensors, 3D nanostructuring, etc.). The kinetics of self-assembled monolayer formations have been reported for several types of reactive groups (trimethoxysilanes, thiols, etc.).
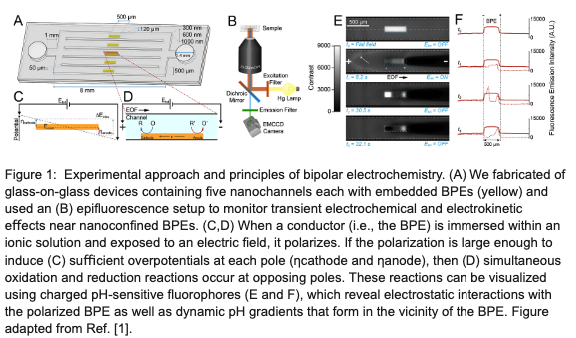
To probe the behavior of nanoconfined bipolar electrodes, we have performed preliminary experiments in which we monitored electrochemical dynamics in real-time using charged fluorophores. Through analysis of fluorescence enhancement and pH-sensitive quenching events, our results indirectly reveal the electrokinetic and electrochemical phenomena that occur near a nanochannel-confined BPE as it becomes charged, reaches a quasi-steady operating state, and then discharges upon the removal of an applied field.